Application dossier
Application dossiers are the collections of technical documents supporting your application. You need to submit all documents in English.
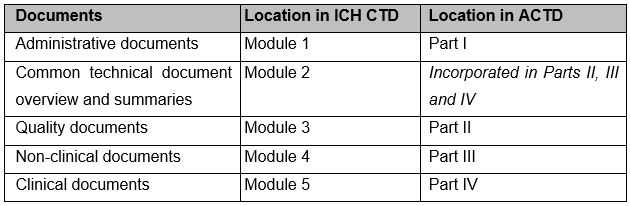
Your application dossier must be organised in either an International Council for Harmonisation Common Technical Document (ICH CTD) or ASEAN CTD (ACTD) format. These formats use the modular framework described by the ICH Topic M4 and the ASEAN guidelines on the Common Technical Document for Registration of Pharmaceuticals for Human use: Organisation of the Dossier, respectively.
The table below summarises the organisation of each dossier format:
Application checklist
Application checklists provide guides on the documentary requirements for the respective applications.
Each application must be accompanied by the chosen CTD application checklist, completed by the applicant:
How to apply
Submit your applications through SHARE.