Update on trend of lymphadenitis and injection-site reactions with the BCG vaccine SSI®
HSA would like to update healthcare professionals on the trend of lymphadenitis and injection-site reactions associated with the Bacillus Calmette-Guérin (BCG) vaccine SSI® (DKSH Singapore Pte Ltd). Since our last update in December 2011 on the increase in local cases of suppurative lymphadenitis,1 the incidence of lymphadenitis has been on the downtrend.
In Singapore, BCG vaccine is routinely administered to all neonates at birth as part of the National Childhood Immunisation Schedule (NCIS). The BCG vaccine SSI® containing an attenuated strain of Mycobacterium bovis (Danish strain 1331) has been the only BCG vaccine registered in Singapore since June 2003.
Reports of lymphadenitis
An increase in local cases of BCG-associated suppurative lymphadenitis was first observed in 2011, based on the vaccine adverse event (VAE) reports obtained from the active surveillance sentinel site2 at KK Women’s and Children’s Hospital (KKH) and spontaneous reporting by healthcare professionals. As of end August 2014, the estimated incidences of suppurative lymphadenitis and non-suppurative lymphadenitis for the 2009 to 2013 vaccinated cohorts ranged from 0.48 to 3.18 per 1,000 vaccinees and 0.10 to 0.79 per 1,000 vaccinees, respectively (Figure 1). Although the upper bound of this estimate was higher than that in the package insert, it still remained within the values that had been reported globally for this brand of vaccine. Following a peak in cases reported in the 2011 vaccinated cohort, the incidences of suppurative and non-suppurative lymphadenitis have since returned to baseline levels.
Figure 1. Incidence of lymphadenitis for the 2009 to 2013 vaccinated cohorts
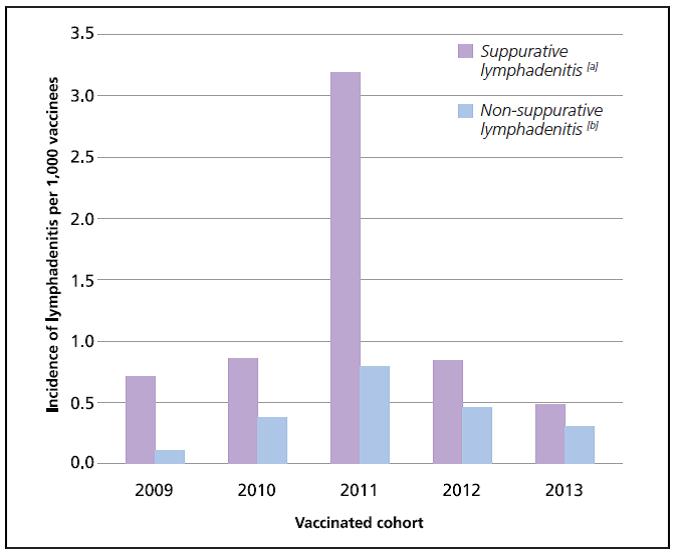
[a] Suppurative lymphadenitis is defined as the presence of fluctuation on palpation or pus on aspiration, the presence of a sinus, or large lymph nodes adherent to skin with caseous lesions on excision.3
[b] The estimates for non-suppurative cases are likely to be underestimates as these cases are usually not reported to HSA given that it is a common and expected reaction post-BCG vaccination.
An investigation into the possible causes behind the higher incidence of suppurative lymphadenitis involving the 2011 vaccinated cohort was conducted by HSA.4 Possible causes such as issues related to vaccine quality, vaccine administration practice or techniques and host characteristics were evaluated in detail. HSA’s investigation revealed that the issue may be batch-related as a result of vaccine manufacturing issues, after ruling out vaccine administration-related and host-related factors.
Further investigation conducted by the manufacturer identified the possible cause to be due to a period of manufacturing with a slower growth of the bacilli. One postulation could be that the higher level of bacterial by-products as a result of the slower growth of the bacilli may have triggered lymphadenitis in some patients, although the manufacturer was unable to confirm this. The trending of the incidence of suppurative lymphadenitis of all batches administered from 2009 to 2013 in Singapore appeared to be consistent with the manufacturer’s investigation, with lower incidences of suppurative lymphadenitis (0.1–1 per 1,000 vaccinees) reported in batches manufactured before and after the period of the slower growth issue. Rectifications made by the manufacturer had seen the incidence of suppurative lymphadenitis returning to baseline levels. Further details may be found in the joint publication by HSA and KKH.4
HSA continues to closely monitor the local VAE reports of BCG-associated lymphadenitis stratified by vaccine batches. Healthcare professionals are encouraged to report the batch number of all vaccines administered according to the NCIS to the National Immunisation Registry, Health Promotion Board, as well as when filing a report of suspected VAE to HSA. This would help in the identification of any quality-related issues with the suspected vaccine during the investigation of VAEs.
Injection-site reactions
HSA also wishes to highlight to healthcare professionals that during our close monitoring of all VAEs associated with the BCG vaccine, neonates who were administered this vaccine at the gluteal area appeared to have experienced more injection-site reactions compared to those who were vaccinated at the deltoid area. A total of 13 cases (0.14 per 1,000 vaccinees) of abscess or cellulitis were reported in children who were vaccinated at the gluteal area compared to six cases (0.10 per 1,000 vaccinees) in those vaccinated at the deltoid area for the 2010 to 2013 vaccinated cohorts. This may be contributed in part by the difficulty in caring for the injection site when the vaccine was administered at the gluteal area. Healthcare professionals are advised to take this into consideration during the administration of the BCG vaccine.
References
1. HSA ADR News Bulletin 2011 Dec; 13: 1-2
2. Vaccine 2014; 32: 5000-5
3. Vaccine 2005; 23: 2676-9
4. Vaccine 2014; 32: 5809-15
Healthcare professional, Industry member, Therapeutic Products
Published:
Safety Alerts