Biotin interference with thyroid function tests
Biotin also known as vitamin B7, is involved in the metabolism of fats, carbohydrates and amino acids required in protein synthesis. It is commonly present in health supplements (such as multivitamins, prenatal vitamins, and products promoting hair, skin and nail growth), and may interfere with thyroid function tests that are based on a biotin/streptavidin interaction. Depending on the assay design, this may lead to either falsely decreased or falsely increased test results, resulting in potential patient mismanagement or misdiagnosis. The risk of interference increases with higher doses of biotin. Healthcare professionals are reminded to consider the possibility of biotin interference when interpreting results of thyroid immunoassays, especially when the results do not match the clinical presentation.
Mechanism behind biotin interference with thyroid function tests
Thyroid function tests measure the levels of thyroid hormones in the blood including thyroxine (T4) and triiodothyronine (T3), as well as thyroid stimulating hormone (TSH). These tests are critical in diagnosing and monitoring thyroid disorders, such as hyperthyroidism and hypothyroidism. Generally, there are two types of thyroid immunoassays used in the measurement of thyroid function. They are the sandwich assay to measure larger molecules such as TSH, and the competitive assay to measure small molecules such as T3 and T4.1 These immunoassays utilise the interaction between biotin and streptavidin (a glycoprotein) as a detection method due to the specific binding between the two biomolecules. Exogenous biotin (e.g., from multivitamins or supplements for hair, skin and nails) can therefore interfere with both types of immunoassays, resulting in either a falsely decreased or falsely increased test result, depending on the assay design.1,2 This potential interaction has been reported with oral products containing ≥ 150 mcg biotin per dose unit and parenteral products containing ≥ 60 mcg biotin per dose unit.3
In a TSH sandwich assay, excess biotin occupies the streptavidin binding sites and prevents the binding of TSH-antibody sandwich complex, causing falsely low assay results (Figure 1A). Conversely, in competitive immunoassays where the endogenous analyte (i.e., T3 or T4) competes with the labelled analyte (i.e., source of the signal) for biotinylated antibody binding sites, excess biotin levels result in falsely high assay results. This is because biotin prevents the binding of antibody-labelled analyte and antibody-endogenous analyte to the streptavidin-coated solid phase. The unbound antibodies are removed in the wash step, thereby removing any signal that indicates the concentration of endogenous analyte present. Since the serum concentration of the endogenous analyte is inversely proportional to the signal intensity, this results in falsely elevated values (Figure 1B).1,4
The interference of biotin with thyroid immunoassays is a known phenomenon and has been documented in several case reports.4 This had resulted in misdiagnosis or clinical mismanagement of thyroid disorders due to the dependence on thyroid function test results for the initiation or adjustment of thyroid medications.
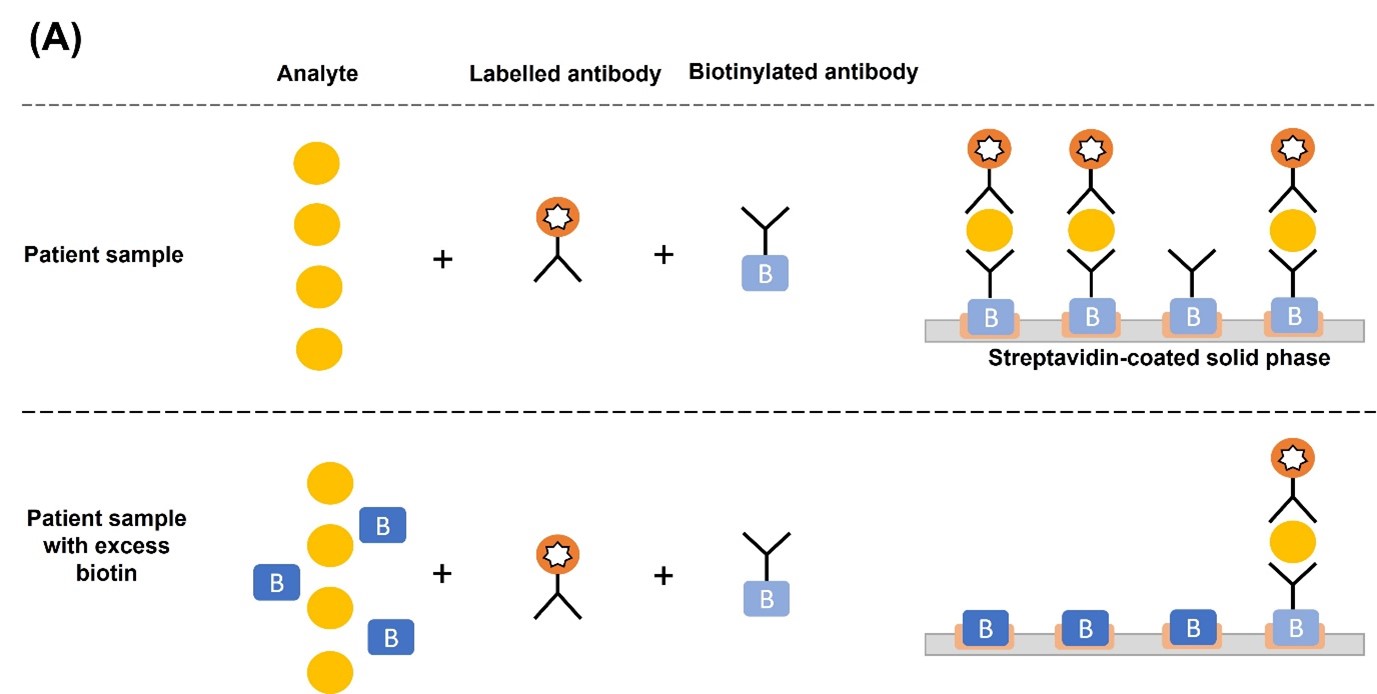
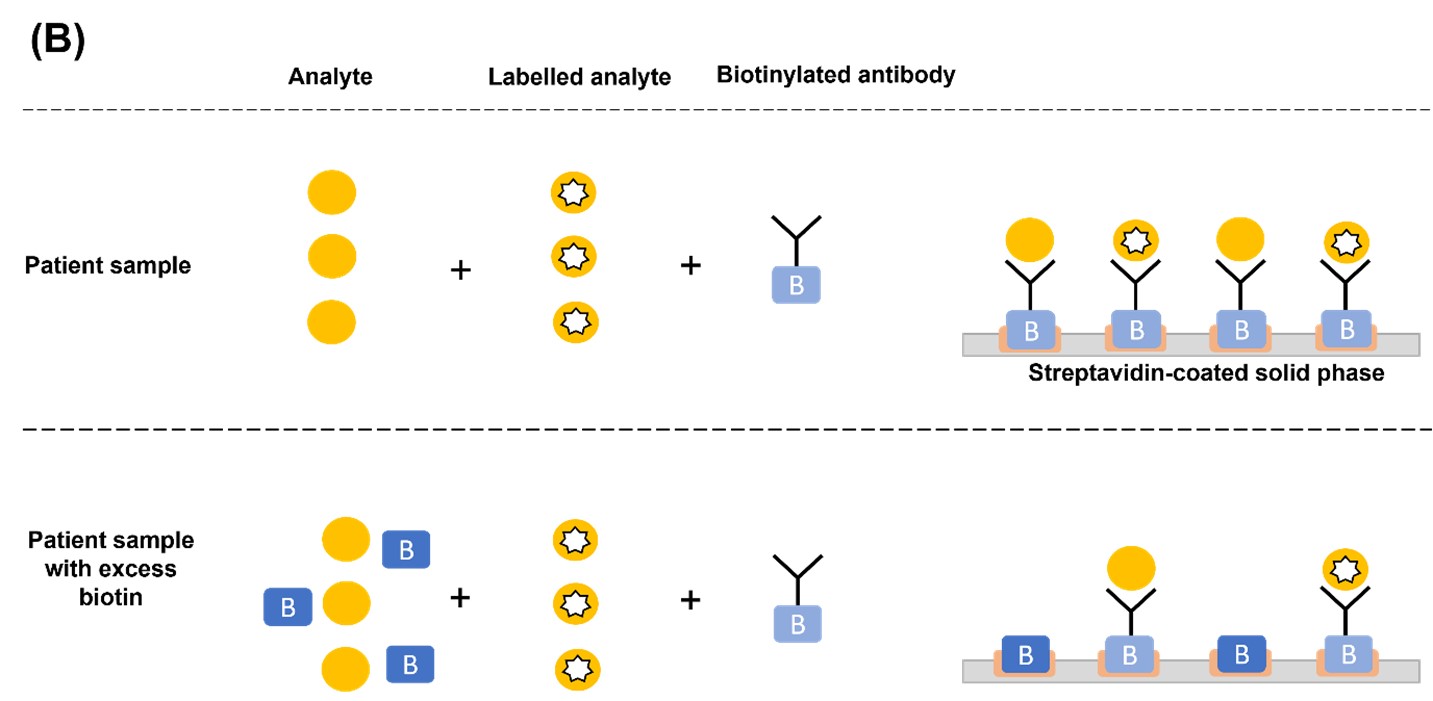
Figure 1. Mechanism of biotin interference in (A) sandwich and (B) competitive immunoassays
International regulatory actions
In November 2022, the European Medicines Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) recommended for the addition of new warnings relating to biotin interference with thyroid function tests to the product information of levothyroxine-containing products. This followed their review on the possible biotin interference with thyroid function tests, which considered the information from spontaneous reports and literature.5
Local situation
In 2019, HSA had assessed the possibility of biotin interference with clinical laboratory tests, including thyroid function tests, and worked with the companies to include warnings on the possible interference in the local package inserts (PIs) of parenteral biotin-containing products. This was communicated to healthcare professionals through an article in the ADR News Bulletin (September 2019) issue.6
In view that this safety concern may also affect patients who are on levothyroxine therapy, HSA has worked with the product registrants to include similar warnings on the possibility of biotin interference with thyroid function tests in the local PIs of levothyroxine products.
To date, HSA has not received any local adverse event reports of biotin interference causing incorrect thyroid function test results.
HSA’s advisory
Healthcare professionals are reminded to consider the possibility of biotin interference when interpreting results of thyroid function tests, especially if there is a lack of coherence with the clinical presentation observed. This may involve asking their patients about the use of biotin health supplements, such as those marketed for hair, skin and nail growth.
References
- J Appl Lab Med 2018; 2: 941-51
- J Clin Lab Anal 2019; 33: e22667
- https://www.ema.europa.eu/documents/prac-recommendation/prac-recommendations-signals-adopted-14-17-january-2019-prac-meeting_en.pdf
- Clin Biochem 2019; 74: 1-11
- https://www.ema.europa.eu/en/documents/psusa/levothyroxine-cmdh-scientific-conclusions-grounds-variation-amendments-product-information-timetable/00001860/202201_en.pdf
- HSA ADR News Bulletin 2019 Sep; 21: 3
Healthcare professional, Industry member, Therapeutic Products
Published:
Safety Alerts