HSA Seized Over 1.12 Million Units of Illegal Health Products and Removed More Than 12,000 Illegal Product Listings Online in 2023
In 2023, the Health Sciences Authority (HSA) continued to clamp down on the sale and supply of illegal health products at targeted hotspots and online. Targeted operations, intel-sharing and joint enforcement efforts with local and overseas agencies led to the seizure of over 1.12 million units of illegal health products and the removal of over 12,000 listings of illegal health products from local e-commerce and social media platforms. These products were unregistered, counterfeit, or had potent medicinal ingredients and/or banned substances illegally added.
Over 50% increase in illegal health products seized
2 Compared to 2022, the number of illegal health products seized by HSA increased by more than 50%. The types of products seized remained similar, with sexual enhancement or male vitality products and addictive medicines such as codeine cough syrup and sedatives, continuing to be the most common categories [Figure 2.1].
Figure 2.1: Types of illegal health products seized in 2022 and 2023
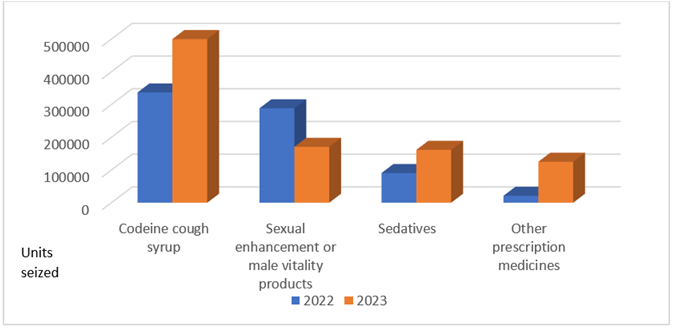
3 The 50% increase in products seized was due to heightened surveillance and enforcement in hotspot areas, including a major operation in June alongside the Singapore Police Force (SPF) to clamp down on a suspected illegal codeine syndicate[1]. This operation was the biggest involving illegal codeine syndicates since 2019. Approximately 190 litres of codeine cough syrup (equivalent to about 2,100 90ml prescription-sized bottles typically dispensed at clinics) and an assortment of pills were seized, making this one of the largest seizures in the past 5 years.
Over 12,000 product listings removed from local e-commerce and social media platforms
4 In 2023, HSA also worked with the administrators of local e-commerce and social media platforms to take down 12,474 listings of illegal health products. This was almost three times higher compared to 4,569 listings removed in 2022. The significant increase was largely due to two international operations led by INTERPOL[2] that targeted the online sale of sexual enhancement products and other health products, which took place in June and October. In addition, HSA utilised technology including e-commerce surveillance tools which enabled offending listings to be identified and taken down much faster.
5 The majority of the removed listings were selling sexual enhancement or male vitality products, hair and beauty products such as anti-hair loss treatment, facial fillers and adulterated skin whitening products, COVID-19 related products such as test kits and symptom-relieving products, weight loss products, contraceptives, and products for management of chronic conditions (e.g., eczema, psoriasis, gout, diabetes, hypertension) [Figure 5.1]. 1,895 sellers received warnings from HSA. Based on the information available in these removed listings, approximately 48% of sellers were based in Singapore.
Figure 5.1: Types of products detected and removed from local e-commerce and social media platforms in 2023

Prosecution actions against the sale and supply of illegal health products
6 From 2021 to 2023, HSA prosecuted 55 persons for the sale and supply of illegal health products. HSA will take stern enforcement actions against anyone who sells or supplies illegal health products. Sellers and suppliers who are selling such products are liable to be prosecuted and if convicted, may be imprisoned for up to 3 years and/or fined up to $100,000.
7 Of the 16 persons prosecuted in 2023, one notable case involved the illegal importing and selling of medicines on Telegram. HSA worked with the Immigration & Checkpoints Authority (ICA) to target the seller’s illegal activities. This culminated in successful operations at Woodlands Checkpoint and a commercial unit in Ang Mo Kio. In total, more than 94,000 units of sedatives and codeine tablets, and approximately 45 litres of cough syrup (equivalent to about 500 prescription-sized bottles) with an estimated street value of about $190,000 were seized. The 35-year-old male seller was sentenced to 30 weeks’ imprisonment.
Illegal health products found to contain potent ingredients and/or banned substances
8 During the past year, HSA issued public alerts on 15 illegal health products detected through adverse event reporting from healthcare professionals, feedback from consumers or from HSA’s surveillance. These products were either adulterated with potent ingredients and/or banned substances, or unregistered.
- The most common adulterants detected were sibutramine (a banned substance) and potent steroids such as dexamethasone, betamethasone and prednisolone. Sibutramine was detected in five products marketed for weight loss, while steroids were found in five products marketed for pain relief or management of chronic conditions like cough and gout.
- Thirteen persons reported adverse effects through their doctors or directly to HSA, after consuming or using one of the 15 illegal health products. Some of them experienced serious adverse effects such as Cushing’s syndrome and Stevens-Johnson syndrome.
- These products were either purchased online, locally from peddlers, or from overseas through friends or relatives, and were marketed to meet various health and/or lifestyle needs.
Please refer to the Annex for the list of these illegal health products, information on the potent ingredients and/or banned substances they contain, and photos of these products.
Serious adverse effects caused by illegal health products
9 Four consumers developed serious adverse effects after taking four products that were tested by HSA to contain dexamethasone and other steroids. One of them, a man in his 30s, had obtained the product “DND Rx9” from an online seller based in Malaysia. He had been taking it for several months to manage his gout, and subsequently developed symptoms of Cushing’s syndrome, a serious steroid-induced condition characterised by a round or “moon face” appearance, and abnormal blood results[3]. His doctor suspected that the product could be adulterated with steroids and reported the case to HSA. Of the remaining three consumers who took products adulterated with steroids, two also developed Cushing’s syndrome, while one had abnormal blood cortisol levels.
10 Another product, “EUZEMA Confidence Revival Cream”, which was sold online, caused a man in his 30s to develop purpura, a skin reaction characterised by purplish red spots due to small bleeds under the skin[4]. The product was marketed to be “steroid-free”, “100% all-natural herbs” and “able to beat eczema for good with this powerful natural formula”. However, HSA tested it to contain betamethasone (a steroid) and very high levels of arsenic, at over 430 times higher than the allowable limits.
11 The man, who had used “EUZEMA Confidence Revival Cream” for a year, shared, “Everyone has a part to play in reporting such products and the adverse effects they may cause to their doctors. I did think it would be inconvenient to report it and perhaps someone else would have done it, but it turned out that wasn't the case.” His doctor, Dr Kuan Ling Yee from the National Skin Centre, cautions, “Understandably, eczema patients hope to explore alternative treatments as they seek a solution for their condition. However, they should bear in mind that they may encounter false information and inaccurate representations of products, especially with the advancement of the Internet.”
Inappropriate use of medicines to improve alertness led to serious skin reactions
12 Three men in their 30s experienced serious adverse effects after taking modafinil or armodafinil inappropriately to stay awake or improve alertness[5]. They developed multiple mouth ulcers and other symptoms like rash, fever and conjunctivitis. Two developed Stevens-Johnson syndrome (SJS), a life-threatening skin condition with blistering and severe peeling of the skin.
13 Modafinil and armodafinil are potent medicines that should only be prescribed by a doctor for medical conditions such as narcolepsy and used under medical supervision. Self-medication with modafinil or armodafinil to stay alert or improve focus can be harmful as it can cause serious adverse effects such as heart problems, hypertension, and psychiatric conditions including anxiety, hallucinations or mania. Serious skin reactions including SJS and toxic epidermal necrolysis (TEN) have also been reported with the use of modafinil and armodafinil, and can lead to hospitalisation, serious complications or even death. Associate Professor Lee Haur Yueh from the Singapore General Hospital, who treated one of the consumers who was hospitalised, advised, “Avoid obtaining medicines from unregulated sources or online platforms and seek medical advice before consuming new medicines.”
14 Associate Professor Chan Cheng Leng, Group Director of HSA’s Health Products Regulation Group, highlights, “Consumers must play a role in safeguarding their own health. Health products such as medicines or health supplements are not like other commodities such as clothes or household devices, where their quality defects can be readily identified by the individual. There is no telling whether a health product is substandard or adulterated with harmful medicinal ingredients, and the adverse impact on the person’s health can be serious and even fatal”.
Consumer advisory
15 Illegal health products remain a threat to public health and safety. As long as there is demand, unscrupulous dealers will continue to manufacture and sell products with promises of quick health fixes. They constantly find new ways and means to profit and take advantage of people’s health concerns but in reality, they have no regard for the health and safety of their buyers.
16 While HSA continues to clamp down on the sale and supply of illegal health products through surveillance and enforcement, it is important for consumers to be the stewards of their own health and safety by being aware of the dangers of illegal health products:
- False claims and harmful ingredients – Illegal health products are often falsely promoted to be “natural”, “herbal” or have quick or miraculous results. In fact, they contain undeclared potent ingredients which can cause serious adverse effects when used in the absence of medical supervision.
- Lack of quality controls, manufacturing and product information – There is no knowing how these products were made, what ingredients they contain and under what conditions they were manufactured and stored.
- No recourse for consumers – It can be difficult to determine the source of products purchased from unknown or unfamiliar sellers such as from overseas, online or street peddlers. Consumers may therefore not be able to claim for any damages or get any refund should anything go wrong.
17 It is not possible to know for sure whether a health product is illegal or harmful just by looking at a product or its packaging. Hence, consumers are advised to always be wary and follow the A-B-C-D steps before buying or taking a health product:
- Avoid making purchases from suspicious or unfamiliar sources. Find out who you are buying from and what you are buying, even when the product is recommended by someone you trust. As a general guideline, buy from reputable sources such as pharmacies or established retail stores. When purchasing from e-commerce platforms or over the internet, consumers are strongly encouraged to purchase from businesses with established retail presence.
- Beware of deals that sound too attractive. If the price is much lower than expected, or product information sounds too good to be true, it probably is.
- Check the claims and products. Not all advertised claims are true and they can be exaggerated or over-promise. Do not be misled by positive product testimonies or reviews online as they cannot be verified. Consumers may also refer to HSA’s database of notified health supplements and traditional medicines (https://www.hsa.gov.sg/vns-list) before making their purchases.
- Discuss with your doctor or pharmacist on the suitability of a product if you are unsure.
18 Members of the public who have any information on the sale and supply of these illegal products may contact HSA's Enforcement Branch at Tel: 6866-3485 during office hours (Monday to Friday) or email: hsa_is@hsa.gov.sg.
HEALTH SCIENCES AUTHORITY
SINGAPORE
30 JANUARY 2024
Consumer, Healthcare professional, Industry member, Chinese Proprietary Medicines, Cosmetic products, Health supplements, Homeopathic medicines, Medical devices, Therapeutic Products, Traditional medicines
Published:
Press Releases