Risk of falsely elevated oestradiol levels due to cross-reactivity of fulvestrant with oestradiol immunoassays
HSA would like to highlight to healthcare professionals about the risk of falsely elevated oestradiol (E2) levels due to cross-reactivity of fulvestrant with E2 immunoassays, which can result in unnecessary therapy modification.
Fulvestrant (Faslodex®, AstraZeneca Singapore Pte Ltd) has been registered in Singapore since 2006. It is indicated for the treatment of post-menopausal women with oestrogen receptor (ER)-positive, locally advanced or metastatic breast cancer for disease relapse on or after adjuvant anti-oestrogen therapy, or disease progression on therapy with an anti-oestrogen. Fulvestrant is a competitive ER antagonist with an affinity comparable to E2. Its mechanism of action is associated with down-regulation of ER protein levels.
Background1
Medical and scientific literature as well as rare international post-marketing reports suggest that fulvestrant can cross-react with E2 immunoassays due to its structural similarity with E2. This can result in falsely elevated E2 levels, which could potentially lead to unnecessary surgery or endocrine therapy modification due to misinterpretation of menopausal women as being premenopausal.
Case report on falsely elevated E2 levels in a postmenopausal patient2
A 36-year-old woman with ER-positive breast cancer underwent bilateral oophorectomy, followed by treatment with letrozole and fulvestrant. The patient was later found to have an unexpected increase in E2 level which was inconsistent with the menopausal symptoms she experienced, such as hot flushes. A pelvic ultrasound revealed a possible small soft tissue density in the left adnexal region that was suspected to be residual ovarian tissue persisting after oophorectomy (a rare condition known as ovarian remnant syndrome). The patient subsequently underwent additional imaging and surgical intervention with diagnostic laparoscopy to remove the possible remnant. Pathology however, revealed no ovarian tissue, thus ruling out ovarian remnant syndrome as the cause of her increased E2 levels.
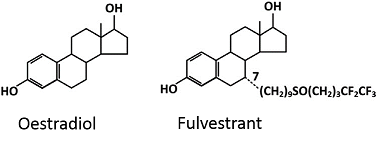
Testing of the patient’s serum E2 levels using a more sensitive and specific liquid chromatography-tandem mass spectrometry (LC-MS/MS) method showed the patient’s serum E2 levels to be undetectable, hence confirming that an exogenous agent was likely to be cross reacting with the immunoassay and producing falsely elevated serum E2 readings. Fulvestrant possesses the basic structure of E2 with the exception of an aliphatic chain of 14 carbons at position 7α (Figure 1). This allows the drug to bind anti-E2 antibodies in the immunoassay and cause false positive results. Based on this structural similarity, fulvestrant was deemed the most likely culprit that led to the falsely elevated E2 reading in this patient.
Actions by other regulatory agencies
Health Canada has published an advisory regarding the risk of unnecessary therapy modification due to falsely elevated E2 levels in patients taking fulvestrant.2 Apart from issuing a Dear Healthcare Professional Letter in Canada, AstraZeneca has also updated the Faslodex® product monograph to include warnings about this safety issue.
The Australian Therapeutic Goods Administration (TGA),3 European Medicines Agency (EMA)4 and United States Food and Drug Administration (US FDA)5 have also updated the product labelling of Faslodex® with warnings that fulvestrant can interfere with E2 measurement by immunoassays, resulting in falsely elevated E2 levels.
Local situation
To date, HSA has not received any local ADR reports of falsely elevated E2 levels associated with the use of fulvestrant. HSA is working with AstraZeneca to update the local Faslodex® package insert to reflect information on the drug-mediated cross-reactivity of fulvestrant with E2 immunoassays.
HSA’s advisory
Healthcare professionals are advised to indicate if their patient is on fulvestrant when requesting blood tests that include E2 levels and to consider alternative methods such as liquid chromatography-mass spectrometry instead of immunoassays to detect E2. Healthcare professionals may also consider the need to carry out a review of the previously reported E2 test results in patients on fulvestrant.
References
- http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2016/60590a-eng.php
- Clin Breast Cancer 2016; 16:e11-3
- Faslodex® Australian Product information, updated on 8 August 2016
- Faslodex® Summary of Product Characteristics, updated on 9 November 2016
- Faslodex® US Product label, updated on 7 December 2016
Healthcare professional, Industry member, Therapeutic Products
Published:
Safety Alerts